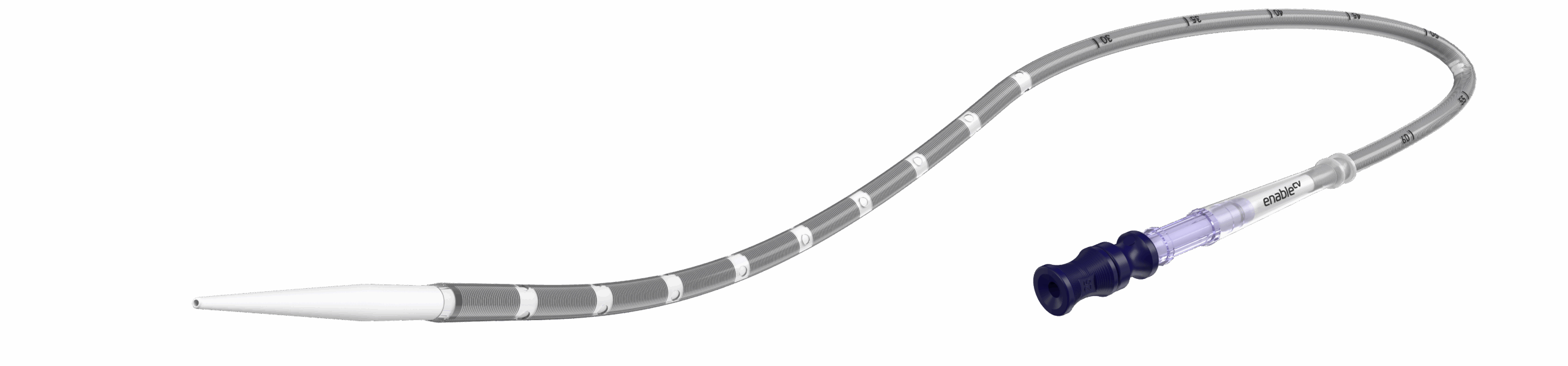
QuickDraw Plus
femoral venous cannula
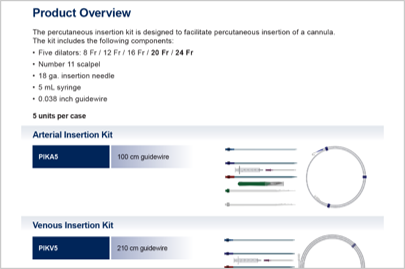
PERCUTANEOUS insertion.
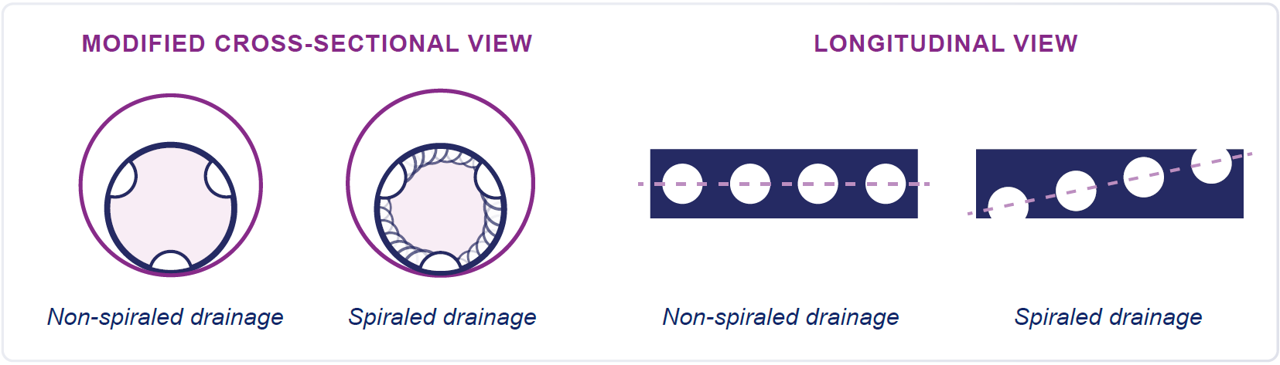
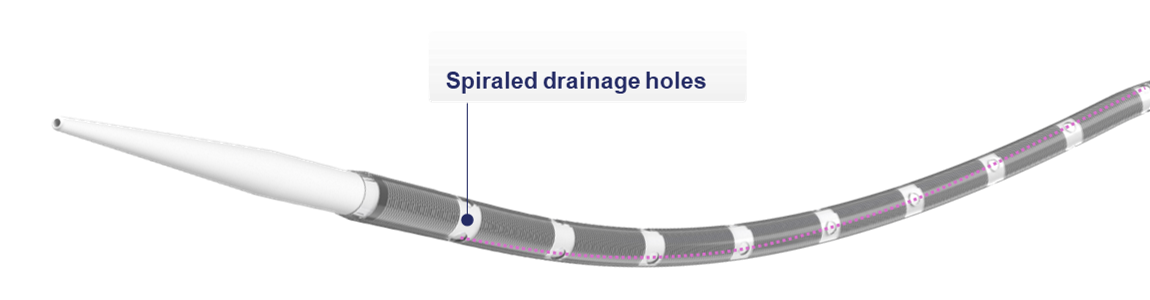
SPIRALED drainage.
OPTIMIZED flow.
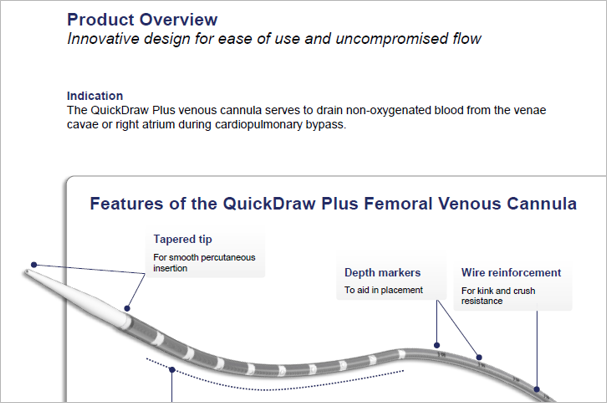
Innovative design for ease of use and uncompromised flow1

PERCUTANEOUS INSERTION
The QuickDraw Plus is engineered to facilitate simplified percutaneous insertion.
SMOOTH
- A smooth tip transition between introducer and cannula combined with a tapered dilator
ACCURATE
- Clearly defined numerical depth markers guide placement
LOCKED
- An innovative single-piece introducer features a unique locking mechanism

SPIRALED DRAINAGE
Engineered with proprietary spiraled drainage holes, QuickDraw Plus is designed for reliable performance with less risk of side occlusion.
RELIABLE
- Drainage holes are strategically rotated around the cannula for reliable, spiraled drainage performance
EXPANDED
- Increased drainage hole surface area vs. original QuickDraw
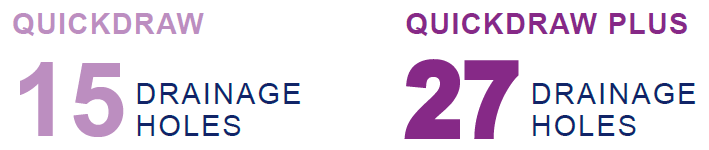

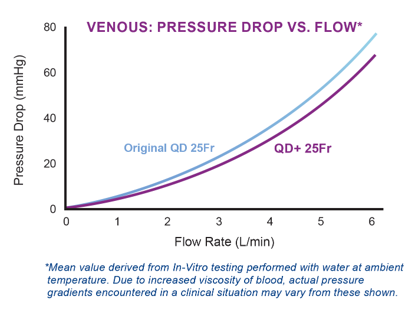
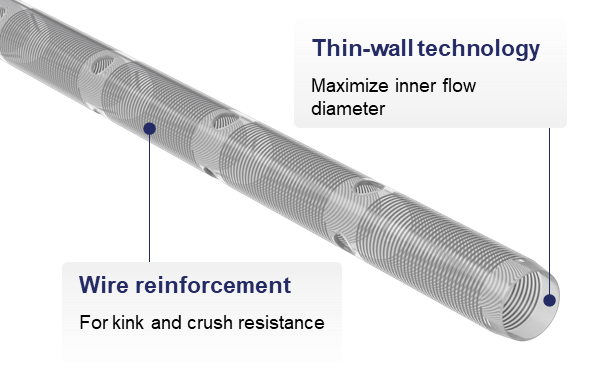
OPTIMIZED FLOW
Thin-walled technology optimizes flow while maintaining crush and kink resistance.
THIN-WALLED
- Maximizes inner diameter (ID) without increasing outer diameter (OD) for optimal flow
CRUSH-RESISTANT
- Reinforced wire-wound body designed to preserve flow rate during surgical manipulation
KINK-RESISTANT
- Engineered cannula resists kinking for ease of use during manipulation
QuickDraw Plus Femoral Venous Cannula
Technical Specifications
| Model # | QDP25 |
|---|---|
| Cannula Spec |
|
| Package Contents |
|
Note: Insertion components are not included.
PIKV5 Percutaneous Insertion Kit is recommended.
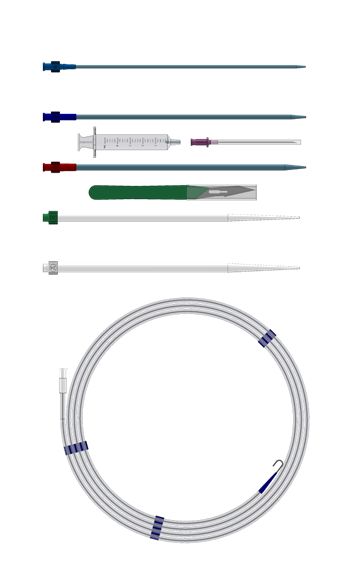
Percutaneous Insertion Kit – Venous Insertion
Technical Specifications
| Model # | PIKV5 |
|---|---|
| Package Contents |
|
Note: PIKV5 Percutaneous Insertion Kit is not included with QuickDraw Plus cannula.
References
1. enableCV bench data on file, may not be indicative of clinical performance.
Stay Connected
Please fill out this form to receive the latest information and updates from enableCV.